In the era of big data and precision medicine, healthcare organizations are increasingly seeking ways to standardize and harmonize their data for research and analytics. The Observational Medical Outcomes Partnership Common Data Model (OMOP CDM) has emerged as a leading standard for transforming disparate healthcare data into a consistent, research-ready format. This article explores the essential steps to implement OMOP CDM, offering practical insights, real-world examples, and best practices for a successful transformation.
Understanding OMOP CDM
OMOP CDM is a standardized data model developed by the Observational Health Data Sciences and Informatics (OHDSI) community. It enables the systematic analysis of disparate observational databases by mapping them to a common structure and vocabulary. This harmonization facilitates large-scale analytics, cross-institutional research, and the generation of real-world evidence.
- Interoperability: OMOP CDM allows data from different sources—such as electronic health records (EHRs), claims, and registries—to be analyzed together.
- Scalability: The model supports millions of patient records, making it suitable for national and international studies.
- Community Support: A vibrant global community provides tools, resources, and shared knowledge.
Key Steps to Implement OMOP CDM
1. Assess and Profile Your Source Data
Begin by thoroughly understanding your source data. This involves profiling the data to identify its structure, content, and quality. Tools like OHDSI’s Data Quality Dashboard can help assess completeness, conformance, and plausibility.
- Identify data sources (EHR, claims, registries, etc.)
- Document data elements and their formats
- Evaluate data quality and gaps
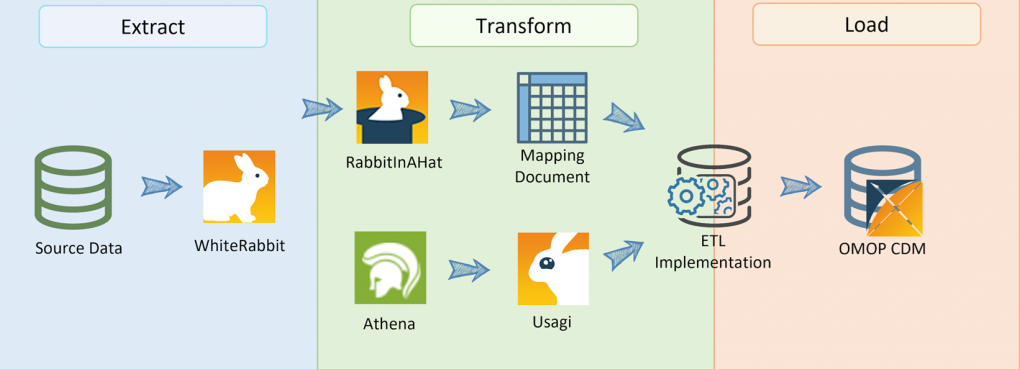
2. Map Source Data to OMOP CDM Structure
Mapping is the most critical and resource-intensive step. It involves aligning your source data tables and fields to the OMOP CDM schema, which includes tables like Person, Observation, Condition Occurrence, and more.
- Use tools like WhiteRabbit and Usagi for data scanning and vocabulary mapping.
- Engage clinical and technical experts to ensure accurate mapping.
- Document all mapping decisions for transparency and reproducibility.
3. Transform and Load Data (ETL Process)
The Extract, Transform, Load (ETL) process converts your mapped data into the OMOP CDM format. This step often requires custom scripts or ETL tools tailored to your data environment.
- Develop ETL pipelines using SQL, Python, or ETL platforms.
- Validate transformations with sample data and edge cases.
- Iterate and refine based on feedback and testing.
4. Standardize Vocabularies
OMOP CDM relies on standardized vocabularies such as SNOMED CT, RxNorm, and LOINC. Mapping local codes to these standards ensures semantic interoperability.
- Leverage OHDSI’s vocabulary mapping tools.
- Address unmapped or ambiguous codes with expert review.
- Regularly update vocabularies to maintain alignment with the latest standards.
5. Conduct Data Quality and Validation Checks
After loading data into OMOP CDM, rigorous quality checks are essential. The OHDSI community provides tools like Achilles and the Data Quality Dashboard for automated validation.
- Check for missing or inconsistent data.
- Validate against source records and known benchmarks.
- Document and resolve any issues before proceeding to analysis.
Case Study: OMOP CDM Implementation at Columbia University
Columbia University Medical Center successfully transformed over 6 million patient records from their EHR system into OMOP CDM. By leveraging OHDSI tools and engaging multidisciplinary teams, they achieved:
- Improved data quality and consistency across departments
- Participation in multi-center studies, such as COVID-19 research
- Faster generation of real-world evidence for clinical decision-making
Their experience highlights the importance of strong project management, stakeholder engagement, and iterative validation throughout the transformation process.
Challenges and Best Practices
Implementing OMOP CDM is not without challenges. Common hurdles include data heterogeneity, resource constraints, and evolving standards. However, organizations can mitigate these by:
- Building cross-functional teams with clinical, technical, and data expertise
- Starting with pilot projects before scaling up
- Engaging with the OHDSI community for support and shared solutions
- Investing in training and documentation
Conclusion: Unlocking the Power of Standardized Data
Transforming your data to OMOP CDM is a strategic investment that unlocks the potential for large-scale analytics, collaborative research, and improved patient outcomes. By following a structured approach—assessing source data, mapping to the CDM, executing robust ETL processes, standardizing vocabularies, and validating quality—organizations can overcome challenges and realize the full benefits of data harmonization. As demonstrated by leading institutions, OMOP CDM paves the way for innovation and evidence-based healthcare on a global scale.